# Neurological basis of parental care
*This writeup is still work in progress. If you have any suggestions/comments/feedback, feel free to share either as comments here or reach out to me by email or on any of the slacks I'm on.*
The following is a summary of what I've learned (so far) about the neurological basis of parental care as a potential case study of a human value. The main source I used for learning all of that is [Michael Numan's *The Parental Brain: Mechanisms, Development, and Evolution*](https://global.oup.com/academic/product/the-parental-brain-9780190848675?cc=us&lang=en&). (Right now, there are not many explicit references in the text but I will probably add them in the future.)
The summary is followed by my attempt to relate it to Steve Byrnes' model of the brain introduced in his [Intro to Brain-Like-AGI Safety sequence](https://www.lesswrong.com/s/HzcM2dkCq7fwXBej8).
## The care circuit
The most important circuit underlying parental care^[I'm generally talking about "parental" rather than specifically "maternal" care because it turns out that the circuits which initially evolved for taking care of the offspring by the mother were later reused/exapted for the same purpose in fathers and, even more generally, [alloparents](https://en.wikipedia.org/wiki/Alloparenting).] is a collection of connected nuclei that I will call "the care circuit". Its main hub is the medial preoptic area (MPOA), which is a nucleus of the hypothalamus essential for any parental behavior. Lesioning MPOA prevents both the onset and maintenance of parental behavior. For example, if you destroy it in a pregnant rat, she will not take care of her children after birth; if you do it in a postpartum rat, she will abandon them (at best). MPOA elicits parental behavior primarily by releasing the ventral pallidum (VP)—the main "output point" of parental behabior—from GABAergic inhibitory projections of the nucleus accumbens (NA). This is done indirectly, by activating the dopaminergic (DA) neurons in the ventral tegmental area (VTA; henceforth VTA-DA), which in turn inhibit NA, thus dinhibiting VP. This is shown on the diagram below.

However, MPOA does not act in isolation. It is activated by various kinds of infant-related stimuli, some of them coming through its dedicated sensory channels and some coming from associative sensory cortex. Infant-related stimuli also arrive to the circuit via the basolateral and basomedial amygdala (BLA/BMA, respectively), activating both NA and activate both the NA and VP. We can view it as giving VP a signal to fire but at the same time adding pushing on the brake (NA) that can be released only when a proper signal comes from MPOA. What happens after VP disinhibition is not clear but studies seem to suggest that the periaqueductal gray (PAG) is somehow involved, though what role it plays exactly is hard to determine.^[PAG is a highly heterogeneous region with different subpopulations of neurons doing very different things. Some of them are definitely involved in parenting-related behavior, such as anxiolysis, aggression towards intruders, and even towards pups in absence of activated maternal motivation. Evidence for PAG's involvement in specific care-related activities is slim but there is a study suggesting involvement in controling the posture when feeding rats.]
Enough abstract neural boxes and arrows. What do I mean by parental behavior, infant-related inputs, and so on?
Take a rat mother taking care of her pups. She feels their characteristic smell, feels them moving under her belly, suckling her nipples, hears ther squeaking etc. These are all examples of inputs to her MPOA and amygdala,^[BLA/BMA to be specific but we'll stick with "amygdala" for simplicity.] which make her lick and groom her children, bring them back them whenever an experimenter removes one of them from her and so on.
What does maternal behavior of a typical mammalian mother look like? Adults of most mammalian species are by default indifferent to their conspecific infants, at best. At worst, they are infanticidal. MPOA thus is not active even in most mammalian females by default. The most usual way it becomes activated is by a sequence of physiological events, which make it more responsive to infant-related stimuli. Numan uses the term "opening the MPOA".^[It is possible that other parts of the circuit also undergo such "opening up", though it's much less clear]. The most important factor involved in the opening up is hormonal stimulation by estradiol and progesterone, which together increase the expression of oxitocin receptors on MPOA and some other nuclei in the care circuit. Around the time of birth, oxytocin is released into the circuit, stimulating its activity, eliciting parental behavior from the VP and enabling the strengthening of the connections between the amygdala and VP circuits, which is enough to make infant-related stimuli sufficient for eliciting parental behavior and oxytocin no longer strictly necessary for their maintenance.^[Injecting oxytocin antagonists or lesioning the PVN (the main source of brain OT) does not disable parental behavior in nonstressful environments, although can lower its quantity and quality.] However, OT does maintain parental behavior in stressful environments, which suppress the care circuit via hormones like the corticotropin-releasing factor (CRF). CRF and OT can be, at least in the limited context of parental care, viewed as counteracting forces fighting each other.
But humans are not infanticidal, right? Most of us don't even have strong aversion toward human infants. And I remember my male dog spontaneously starting to lick a puppy when we encoutered it on a walk. Indeed, the difference in the degree to which different species (and to a lesser extent, members of any particular species) treat unrelated conspecific infants is one of the main variables in parental behavior. Some species have their MPOA more open by default. Female lab mice are an extreme example: they treat any encountered pups of their own species like their own children, even having never been pregnant. This contrasts with their feral counterparts, which are infanticidal. A similar difference, though to a lesser extent, is observed in lab rats versus wild rats, perhaps pointing to some laboratory version of [the domestication syndrome](https://en.wikipedia.org/wiki/Domestication_syndrome). This is in line with the hypothesis of [human self-domestication](https://en.wikipedia.org/wiki/Self-domestication#In_humans) and the fact that humans are the only great apes practicing [alloparenting](https://en.wikipedia.org/wiki/Alloparenting).
However, domestication (or something like it) is not the only way for evolution to open the MPOA up. 5% of mammalian species exhibit biparental care (i.e. fathers take care of their children at least to some extent) and 3% are alloparental (i.e. group members other than the parents participate in caretaking).^[What evolutionary pressures lead to evolution of paternal and alloparental caretaking of the young is an interesting question but beyond the scope of this post.] Obviously, parental circuits in fathers can't be activated by pregnancy and the same is true for many of the alloparents, since the most common alloparent is an older sibling, which has not had their own offspring yet. Therefore, either some stimuli other than pregnancy must activate MPOA or it must be more open by default. In most cases, it's probably a mixture of both.
Lab are an example. Unlike their feral cousins, they are not infanticidal by default, but rather merely avoidant towards conspecific infants. Moreover, both female and male lab rats can be sensitized by exposure to infants, eliciting in them parental behavior in absence of pregnancy or hormonal treatment. On average, this process takes about 7 days in females and 11 days in males.
Apparently, the possibility of exhibiting parental care lies dormant in adult members of at least many mammalian species and needs only some elicitation by relevant stimuli. Interestingly, destruction of a particular sensory channel in rats—in this cases accessory olfactory bulb—does not eliminate or even disturb the sensitization but, quite to the contrary, shortens it.
This is a good time to introduce the bad twin of the care circuit, which I term the "anti-care circuit".^[Numan uses the term "defensive circuit".] This circuit, in turn, is a good starting point for discussing yet other circuits that are also relevant for parental behavior and allow us to see the broader picture within which parental behavior sits.
## Other circuits
### Anti-care
The default aversion/aggression towards infant conspecific exhibited by a majority of mammalian species appears to be implemented by a particular circuit, which is downregulated during pregnancy, while the care circuit (described in the previous section) is upregulated. This anti-care circuit makes the animal aversive (to varying extent) to conspecific young and its downregulation appears to be one of the main ways used by evolution to open MPOA up, allowing for parental behavior in situations which do not involve pregnancy (the other being of course upregulating MPOA). In lab rats, the anti-care relies exclusively on odors coming through the accessory olfactoy tract, which is why ablating that sensory channel can shorten the sensitization latency. The two parts of the central nervous system that are most strongly implicated in this behavior are the central amygdala and the ventro-lateral part of the ventral medial nucleus (VMNvl) of the thalamus (the latter being involved in all kinds of fear/anxiety/defense-related behaviors).
### Anxiolysis
Animals that are in the process of taking care of their young also seem to be generally less fearful and anxious, not only when that fearlessness is helpful for taking care of their offspring (e.g. when defending against an intruder is required; see next section) but also more generally. Interestingly, at least in lab rats and mice, it appears to be contingent on the presence of particular infant-related stimuli. Maternal lab rats lose their fearlessness completely about 4 hours after they have been separated from their pups and. Their fear/anxiety^[The technical distinction drawn between fear and anxiety in neurobiological literature is that fear is directed towards the stimuli that are directly present or perceived whereas anxiety is directed towards the things that are not observed directly but whose presence is predicted or inferred. It is not relevant for this post, so I gloss over it.] levels rise back thhen to those typical of a non-maternal female. However, as soon as she is reunited with her children, her "courage" returns almost immediately. What appears crucial here, is ventral somatosensory stimuli coming from the pups: smell and sounds do not seem to play a significant role. In lab mice, the specificity is even greater in that what is needed is specifically the stimuli of the infants suckling their mother's nipples.
### Parental aggression
Numan hypothesizes that the levels of fear/anxiety exhibited by a properly fine-tuned mammalian female lie in a sweetspot that allows her to appropriately react to intruders being potential danger to the young. Too little fear/anxiety makes her not react when she should. Too much and she flees leaving her young defenseless to the predator or infanticidal conspecific.
Thus, the two systems are depend on each other to some extent. However, they are also in principle dissociable and it is possibly to intervene in one, while disrupting the other only to a limited extent. Parental aggression can be disabled by intervening at any point along the chain from the aggressor-related input to the aggressor-directed output. In rats, that input is primarily (if not exclusively) olfactory and routed through the medial amygdala and the ventral premamillary nucleus of the hypothalamus, eventually ending up in the VMNvl, the nucleus that implements infant-directed aversion in the anti-care circuit. Ablating olfaction in maternal rats removes maternal aggression—if they can't smell the intruder, they don't register it as an intruder.
## Neurology of parental behavior and Steve Byrnes' model of the brain
*This section assumes familiarity with [Steven Byrnes' model of the brain](https://www.lesswrong.com/s/HzcM2dkCq7fwXBej8) (posts 2-7 are the most relevant), distillation of which is obviously beyond the scope of his post. Each subsection includes only a brief recap of relevant pieces of the model.*
### Learning and steering
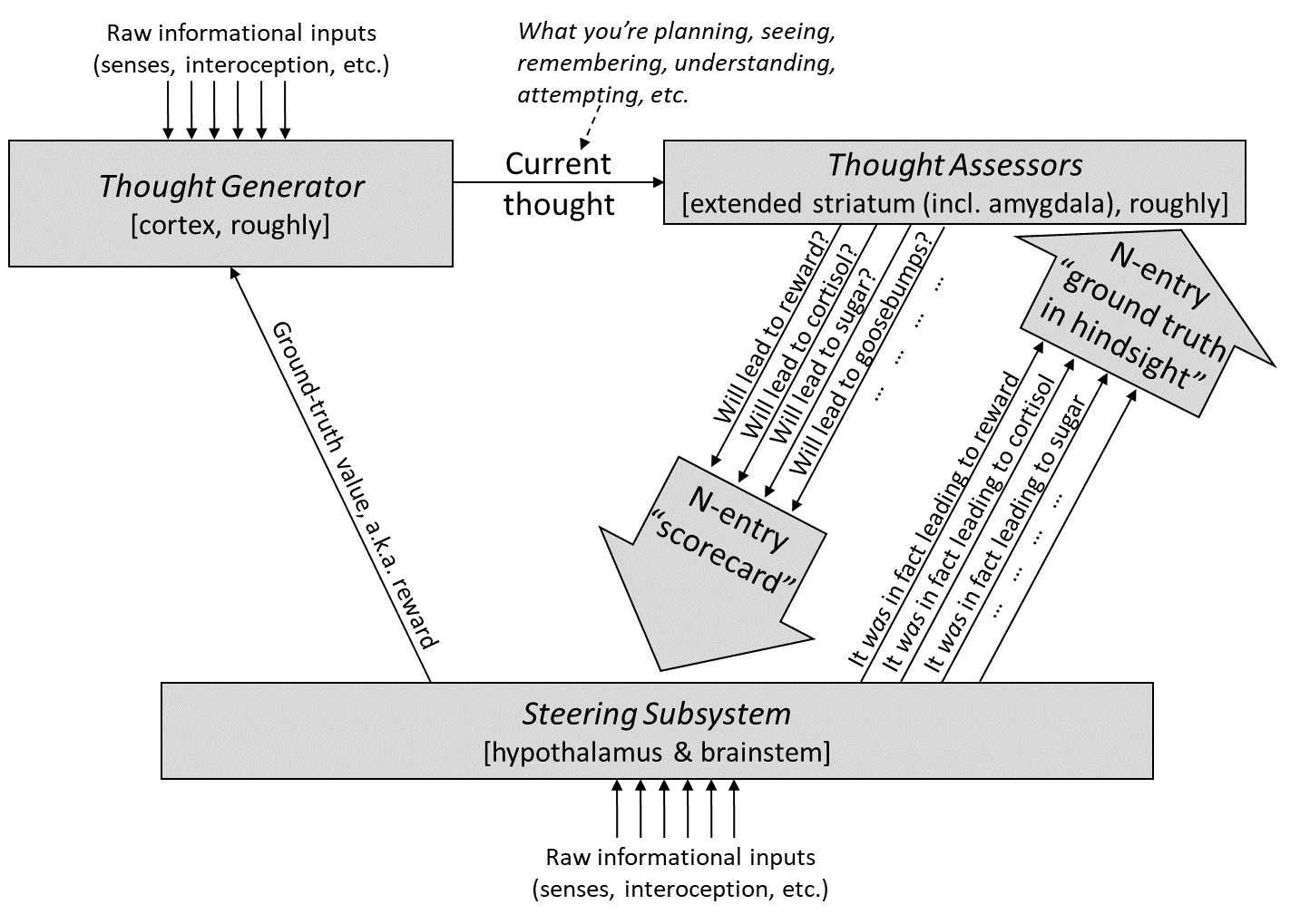
Steve's model [postulates](https://www.lesswrong.com/s/HzcM2dkCq7fwXBej8/p/qNZSBqLEh4qLRqgWW#6_2_2_Quick_run_through) that a finite set of basic drives/motivations (like the ones related to basic physiological needs, mating, social status, etc.) is harcoded in the steering subsystem and that higher/more abstract values that the learning subsystem may learn (e.g., wanting the avoid existential risk and prevent permanently curbing the potential of humanity) are ultimately translated into a format that can be understood by the steering subsystem in order to motivate behavior.
The "(higher-order) values" are represented in what he calls Thought Generators, located in the neocortex, whereas the role of translators is fulfilled by the Thought Assessors, located approximately in the extended striatum (which also includes the amygdala). Can we locate at least some of them on the diagrams of the parental circuits?
The MPOA and VTA are the obvious candidates for the steering subsystem circuits. Both implement hard-coded parenting-related behaviors, although they also have some "innate adjustable parameters" that can change over lifetime (sometimes (semi-)permanently, sometimes not), in agreement with Steve's view described [here](https://www.lesswrong.com/s/HzcM2dkCq7fwXBej8/p/wBHSYwqssBGCnwvHg#2_3_Three_things_that__learning_from_scratch__is_NOT). The most obvious one of them is the variable expression of oxytocin receptors on the neurons of MPOA and VTA (and many other components of the circuit), which increases in response to exposure to particular contexts, such as stimuli related to conspecific infants, or rapidly rising estradiol-to-progesterone level ratio during pregnancy. The MPOA is a part of the hypothalamus, whereas VTA is a part of the brainstem, so this seems to fit Steve's model.
Downstream of the MPOA and VTA, the circuit also contains the nucleus accumbens and ventral pallidum, both of which receive signals from the basolateral and basomedial amygdala (BLA/BMA). The amygdala plus basal ganglia are good candidates for the Thought Assessors and the VTA sends dopaminergic projections to the NA, which fits Steve's accepted view of DA as the reward/ground-truth/prediction error. I am still not quite sure if/how/where all of that fits into Steve's model but at least these few pieces seems to be congruent with it.
### Separate sensory channels?
[Steve postulates](https://www.lesswrong.com/s/HzcM2dkCq7fwXBej8/p/hE56gYi5d68uux9oM#3_2_1_Each_subsystem_generally_needs_its_own_sensory_processor) that the learning subsystem and the steering subsystem have their own separate sensory channels, which allows the hardcoded circuits and the learned circuits process the same ~raw sensory information independently. This mechanism is relevant for [the process of overriding](https://www.lesswrong.com/posts/qNZSBqLEh4qLRqgWW/intro-to-brain-like-agi-safety-6-big-picture-of-motivation#6_4_1_The_cortex_proposes_a__value__estimate__but_the_Steering_Subsystem_may_choose_to_override) the influence of learned long-term predictors by hardcoded steering subsystem circuits.
Does the parental circuit have its own sensory channels? Sensory stimuli enter it in two places: the BMA/BLA and MPOA. Inputs to BLA/BMA are multimodal, coming from the various neocortical regions. Some of them also arrive reach MPOA (especially from mPFC) but MPOA also seems to have its own dedicated channels. At least in the rat case, we have the olfactory signals (e.g. infant odors) which arrive from the two olfactory signals through cortical and medial amygdala, as well as somatosensory stimuli (e.g. infant suckling) that come from the thalamus (more specifically, posterior intralaminar complex; PIL), bypassing the cortex.
So, if we take the view that the MPOA-VTA part of the parental circuit roughly corresponds to the hardcoded part and BLA/BMA+NA+VP rougly corresponds to the Thought Assessor part, this seems to fit Steve's model reasonably well (perhaps with a caveat that there is actually some multisensory cortical input from the learning subsystem entering the steering subsystem).
## Open questions
- What is the relationship between empathy circuits and parental circuits?
- How do deeper reflection and more abstract considerations enter the picture? (e.g. the drowning child example)