# İrem Sultan İlçi / Adeno Associated Virus
:::info
> [name=İrem Sultan İlçi]
>
[Adeno Associated Virus](https://pubmed.ncbi.nlm.nih.gov/32327148/)
:::
# **ADENO ASSOCIATED VIRUSES**
> Adeno-associated viruses (AAVs) are small, non-pathogenic, ssDNA packaging viruses, capable of infecting a wide range of vertebrate hosts, including humans. AAVs belong to the Parvovirinae subfamily of the *Parvoviridae*, and *Dependoparvovirus* genus. As the name implies, they require co-infection with adenovirus or herpesviruses as helpers for replication.
Source: [*Nature/1*](https://www.nature.com/articles/s41467-021-21935-5)
---

Source: [*Frontiersin*](https://www.frontiersin.org/articles/10.3389/fimmu.2014.00009/full)
---
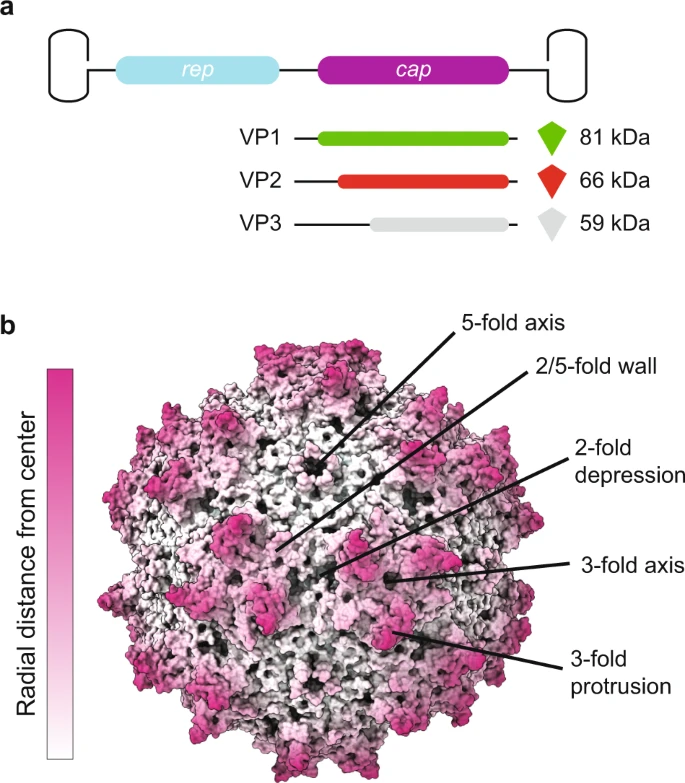
**Capsid Composition**
>The wild type AAVs package a 4.7 kb genome encoding non-structural (rep), structural (cap), assembly activating (aap), and membrane associated accessory (maap) proteins. AAVs have become widely used for gene therapy applications, with several advantages over other viral vectors, including a lower toxicity and the availability of over 150 naturally occurring genotypes and serotypes
---
>
>Source: [*Nature/1*](https://www.nature.com/articles/s41467-021-21935-5)
---
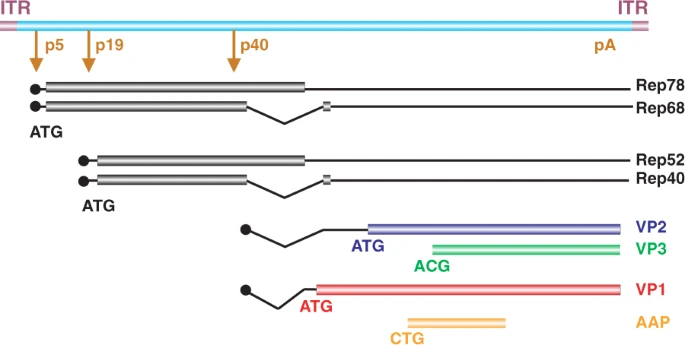
**Wild-type AAV genome organization**
* AAV is a non-enveloped, single-stranded DNA virus with an icosahedral capsid that has a diameter of ~22–26 nm comprised of a total of 60 capsid proteins.
* In the presence of a helpervirus, the Rep proteins play a central role in replication and genome encapsidation; whereas in its absence the large Rep proteins are important for the preferential insertion of the wtAAV2 genome into human chromosome 19.
* The cap gene expresses the three capsid protein subunits, VP1, VP2, and VP3, which have overlapping reading frames in the next slides figure. The ratio of VP1, VP2, and VP3 in the virion is approximately 1:1:10.
* For many, though not all serotypes, the so-called assembly activating protein (AAP), which is expressed from an alternative reading frame within the cap gene, is important for efficient capsid assembly
Source: [*Nature/2*](https://www.nature.com/articles/s41434-021-00243-z)
---
**Wild-type AAV genome organization**
Source: [*Nature/2*](https://www.nature.com/articles/s41434-021-00243-z)
---
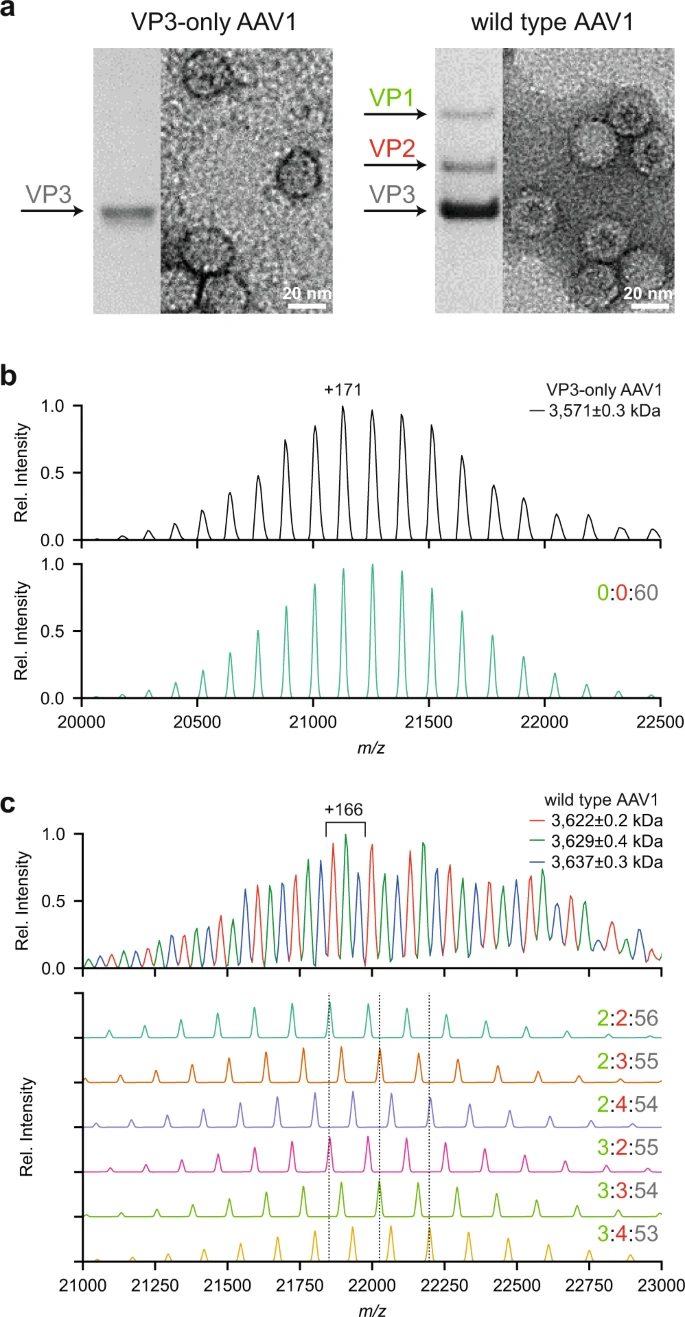
**Capsid Structure and Heterogenecity**
>Here, is an example of AAVs of different serotypes, produced in different facilities, using different system and evaluated whether high-resolution native MS analysis of the empty capsids to determine accurately the mass and exact composition of each of these gene delivery platforms.
---
Source: [*Nature/1*](https://www.nature.com/articles/s41467-021-21935-5)
---
***Design of AAV Vectors for Delivery of Large or Multiple Transgenes***
AAV has a relatively small packaging capacity, and cannot effectively package more than 5 kb of DNA. Obviously, the small packaging capacity of AAV poses a significant challenge to treating genetic diseases with mutations of large genes.
>Multiple strategies have been explored to overcome the size limit of AAV vectors. One strategy is to split large transgenes into two or three parts, generating dual or triple AAV vectors that can deliver large gene expression cassettes. Another strategy is to harness factors that promote the expression of large transgenes from a
single AAV vector.
---
**Design Strategies of AAV Vevtors**
:::info
1. Split AAV Vector
1.1 Trans-splicing AAV Vectors
1.2 Overlapping AAV Vectors
1.3 Hybrid AAV Vectors
1.4 Triple AAV Vectors
2. Oversized AAV Gene Transfer
2.1 Fragmented Oversized AAV Gene Delivery
2.2 Other Strategies
:::
Source: *Michael J. Castle, Adeno-Associated Virus
Vectors Design and Delivery*
---
**Summary of split AAV vectors for delivering large therapeutic genes**

Source: *Michael J. Castle, Adeno-Associated Virus
Vectors Design and Delivery*
---
**Summary of oversized AAV gene delivery**

Source: *Michael J. Castle, Adeno-Associated Virus
Vectors Design and Delivery*
---
***AAV attachment factors and receptors***
:::info
AAV uses multiple attachment factors and receptors.
:::
:::warning
*1. Glycan attachment factors*
Glycan receptors act as important attachment factors mediating initial interaction with the cell.
:::
:::warning
*2. Proteinaceous co-receptors*
Several proteinaceous co-receptors have been identified. While these may enhance transduction in certain cellular contexts, no structural studies have reported direct binding.
:::
:::warning
*3. The AAV multi-serotype receptor*
Recently, the multi-serotype receptor AAVR has been identified and shown to be essential for AAV entry and transduction in a wide range of human cell types and in small animal models.
:::
:::warning
*4. Structural interactions between AAV and AAVR*
AAVR constitutes the major binding activity in cell membranes and structural studies have defined the interaction interface in molecular detail suggesting that AAVR acts as the long sought proteinaceous receptor for AAV. The involvement of glycan attachment factors and an essential receptor suggests a multi-step mechanism of AAV entry.
:::
Source: [Pubmed](https://pubmed.ncbi.nlm.nih.gov/32327148/)
---
Schematic of AAVR:

Source: [Pubmed](https://pubmed.ncbi.nlm.nih.gov/32327148/)
----
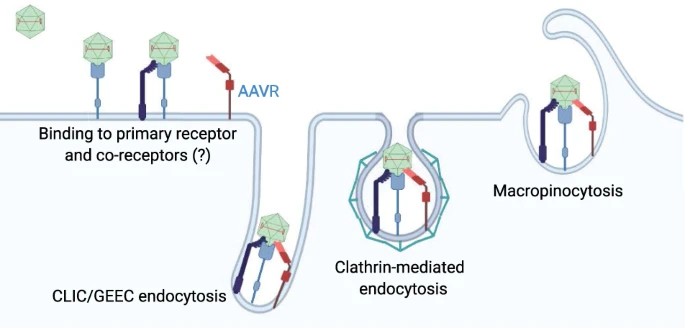
**Attachment and endocytosis**
When AAV reaches the cells, it attaches to receptors/co-receptors, followed by endocytosis into the cell.
:::info
Studies with AAV vectors have shown that the tissue and cell-specific tropism vary widely depending not only on the AAV serotype but also the host species.Furthermore, the route of administration can also have dramatic effects on the AAV tropism.
:::
AAV can follow multiple endocytic routes into the cell:
Source: [*Nature/2*](https://www.nature.com/articles/s41434-021-00243-z)
---
**Trafficking to the Golgi apparatus**
Following endocytosis, AAV must travel to the Golgi for successful transduction:

Source: [*Nature/2*](https://www.nature.com/articles/s41434-021-00243-z)
---
**Trafficking to the Golgi apparatus**
Functional AAV entry requires early passage through acidic endosomes, which results in structural changes in the AAV capsid required for transduction . To further dissect early events in AAV entry, several inhibitors of various trafficking steps were used including an inhibitor of endosome acidification (bafilomycin A), an inhibitor of early to late endosome transition (brefeldin A), and a proteasome inhibitor (MG-132).
Source: [*Nature/2*](https://www.nature.com/articles/s41434-021-00243-z)

Source: [Pubmed](https://pubmed.ncbi.nlm.nih.gov/32327148/)
---
**Escape to the cytoplasm and nuclear import**
* AAV egress from the endomembrane membrane and import to the nucleus.
* After reaching the trans-Golgi network, AAV is released to the cytoplasm presumably by PLA2 mediated escape. The AAV capsid contains multiple proposed nuclear localization signals, which are required for entry through the nuclear pore complex (NPC).
* Once in the nucleus, DNA is released from the capsid and dsDNA is formed. Host factors allow for transcription of the AAV extrachromosomal DNA, producing mRNA that can be translated by host machinery. Some AAV particles can be phosphorylated at tyrosine residues on the capsid by EGFR-PTK.
* This can result in ubiquitination of the capsid followed by degradation in the proteasome. Inhibition of the proteasome leads to increased AAV transduction.
Source: [Pubmed](https://pubmed.ncbi.nlm.nih.gov/32327148/)
---
**Escape to the cytoplasm and nuclear import**
AAV must escape into the cytosol prior to nuclear entry:
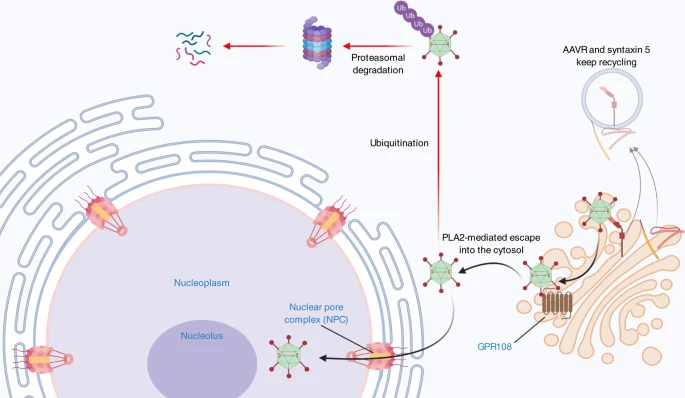
Source: [*Nature/2*](https://www.nature.com/articles/s41434-021-00243-z)
---
**Transgene Expression**
Steps following nuclear import that lead to transgene expression:
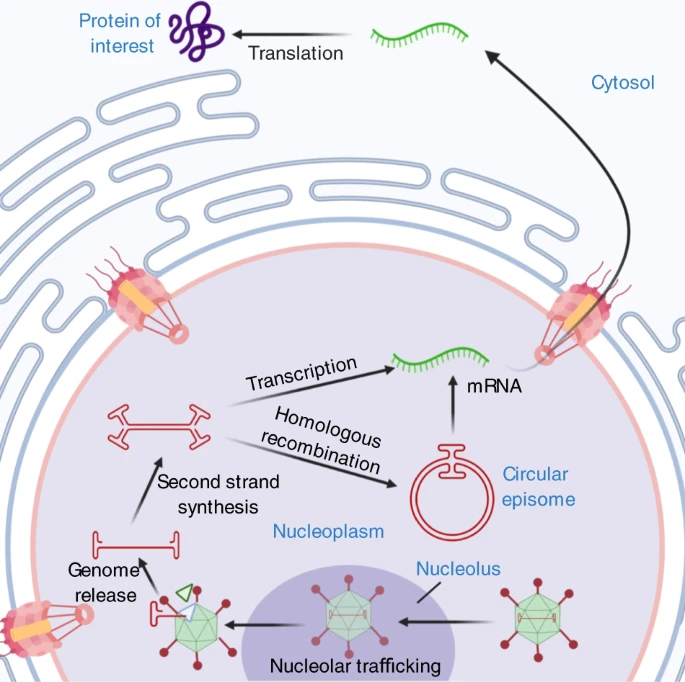
Source: [*Nature/2*](https://www.nature.com/articles/s41434-021-00243-z)
---
**SOURCES**
* [*Nature/1*](https://www.nature.com/articles/s41467-021-21935-5) : Wörner, T.P., Bennett, A., Habka, S. et al. Adeno-associated virus capsid assembly is divergent and stochastic. Nat Commun 12, 1642 (2021). https://doi.org/10.1038/s41467-021-21935-5
* [*Nature/2*](https://www.nature.com/articles/s41434-021-00243-z): Riyad, J.M., Weber, T. Intracellular trafficking of adeno-associated virus (AAV) vectors: challenges and future directions. Gene Ther (2021). https://doi.org/10.1038/s41434-021-00243-z
* [Pubmed](https://pubmed.ncbi.nlm.nih.gov/32327148/): Zengel J, Carette JE. Structural and cellular biology of adeno-associated virus attachment and entry. Adv Virus Res. 2020;106:39-84. doi: 10.1016/bs.aivir.2020.01.002. Epub 2020 Feb 13. PMID: 32327148.
* [*Frontiersin*](https://www.frontiersin.org/articles/10.3389/fimmu.2014.00009/full): Tseng, Y.-S., & Agbandje-Mckenna, M. (2014). Mapping the AAV Capsid Host Antibody Response toward the Development of Second Generation Gene Delivery Vectors. Frontiers in Immunology, 5, 9. https://doi.org/10.3389/fimmu.2014.00009
* Castle, M. J. (Ed.). (2019). Adeno-Associated Virus Vectors. Methods in Molecular Biology. doi:10.1007/978-1-4939-9139-6